| Rotifers as parasites |
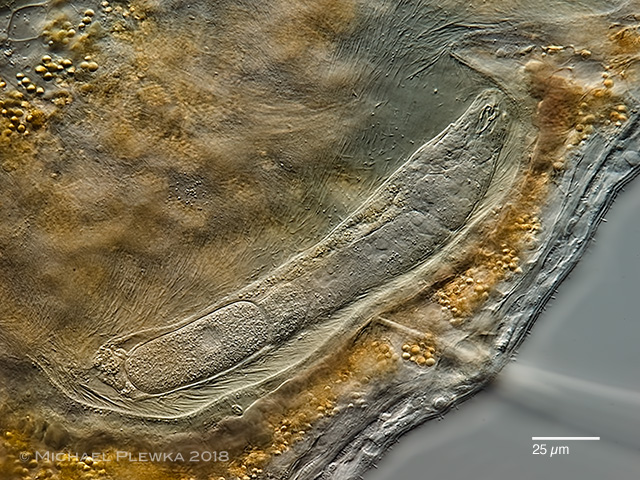 |
| Albertia sp., an endoparasite of Oligochaetes (Nais sp.) |
| |
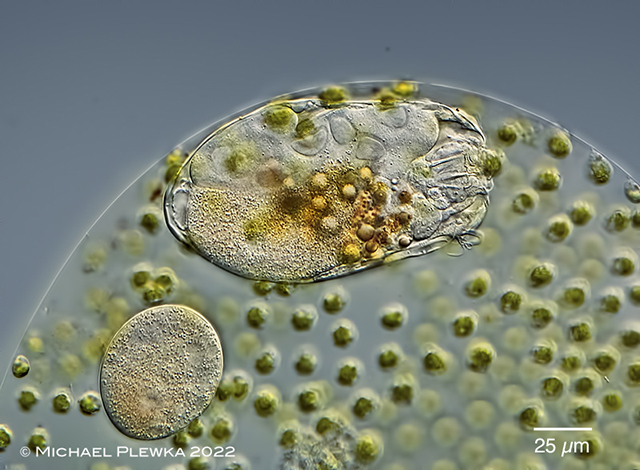 |
| Ascomorphella volvocicola, lives as an endoparasite in the alga Volvox. |
| |
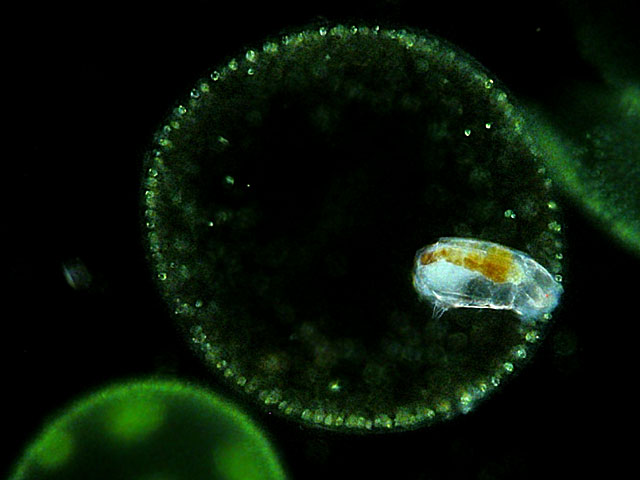 |
| Cephalodella catellina volvocicola, lives as an endoparasite in the alga Volvox. |
| |
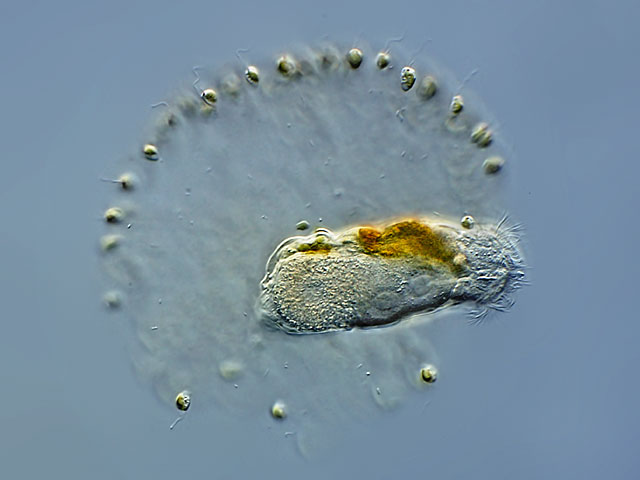 |
| Cephalodella edax, lives as an endoparasite in the chrysophyte alga Uroglena volvox |
| |
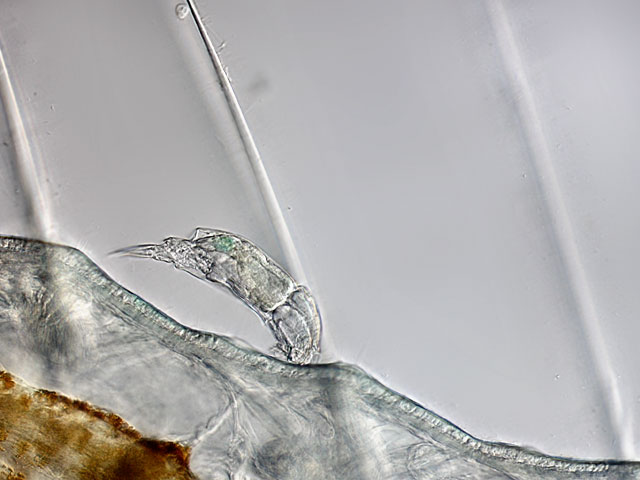 |
| Cephalodella parasitica, an ectoparasite on oligochaetes like Stylaria lacustris or Nais sp |
| |
 |
| Some bdelloid rotifer species are living in the cells of Sphagnum moss. For instance Habrotrocha roeperi (which is depicted in the above image) lives in the retort cells of Sphagnum , whereas Habrotracha reclusa lives in the hyalocytes. Some authors consider this behavior as space parasitism, as well as the behavior of the Bdelloid Mniobia symbiotica, which lives in the lobules of the liverwort Frullania. |
| |
| |
| Rotifers infested by parasites |
| Both taxonomic groups of freshwater rotifers, the Monogononta and the Bdelloidea may be infested by parasites. In principle, rotifers can be infested by both endo- and ectoparasites. The ecto "parasites" are mostly epibiontic organisms such as euglenids or flagellates, which are probably also found on other substrates and are therefore very likely known to science as species. This is not necessarily the case with endoparasites. While the monogonont rotifers have the chance to escape in the liquid water body of ponds and lakes, the bdelloid rotifers, most of which live a thin film of water in mosses or soil, have a different strategy: it seems that they can escape by desiccation. |
| |
| Not many informations exist about the biology and ecology of these endoparasites. One reason for this is that not many biologists bother to look for them or microscopists do not know that a specimen is infested. On the other hand there are researchers looking for infested rotifers and their parasites, but cannot find them because of lack of human resources. |
| So if there should be workshops about the biology/ ecology of microinvertebrates somewhere in the world there might be a chance that rotifer parasites might be observed, but not identified as such. Therefore the images below shall help to identify rotifers that may be infested. |
| In some cases rotifers that are infested appear opaque instead of appearing transparent, so even with a binocular / low magnicification microscope (≈ 30x) an experienced observer can get a hint that "something is wrong" with this specimen. It then could be isolated by transfer to another drop of clean water |
| If one has found such a rotifer specimen infected with endoparasites, one must basically be prepared to have to make a decision regarding the further course of action:
1. identification/documentation
or
2. storage/ cultivation/ molecular analysis
ad 1. identification of the host species on a morphological level, which in some cases requires maceration of the specimen for trophi analysis, so that no subsequent analysis of the parasites is possible.
ad 2. in the event that the host can be identified (or documented by photographs) in the living state, this rotifer should ideally be freed from other organisms (except the parasite) and then dried in an Eppendorf tube. After drying, the eppendorf tube can be closed. The contents can then be used for molecular analyses for at least several months. |
If the infected rotifer has been detected in a Petri dish (or slide without cover slip), it should be transferred to a clean slide using an Eppendorf pipette. This transfer should always be done with an Eppendorf pipette with approx. 25 µl, a Pasteur pipette absorbs far too much water or air, then the critter is lost. Afterwards, the animal/fluid should be freed from other organisms by diluting the drop of fluid with further water low in minerals (e.g. "Volvic" has proven to be very suitable in this case) and then removing other disturbing components with the Eppendorf pipette under observation with the bino. This procedure can be repeated several times if necessary. Then transfer the affected rotifer with the Eppendorf pipette (25µl) into an Eppendorf tube (approx. 1ml) (check with the bino!) and allow it to dry out there (leave the Eppendorf tube open!). After drying, the tube should be closed. The contents can then be used for molecular analyses for at least several months.
This method preserves at least the theoretical chance that the parasites in the dried rotifer might survive as permanent forms and that these could subsequently infect other rotifers.
If you are not sure that the air is free of contamination (spores/pollen etc), you can also add approx. 75µl EtOH 70/ instead of drying and seal it immediately. However, this sample is then only suitable for molecular analysis, but not for further infections. |
| |
| If the infected rotifer with coverslip is under the microscope, the coverslip must first be raised slightly under control in the bino by adding some water ( Volvic ). It is recommended to add water from all 4 sides of the coverslip. Afterwards, the coverslip can be carefully shifted with tweezers until the infected rotifer is exposed and the coverslip can be removed. Cleaning is then carried out in the manner described above.
Since at the present state of knowledge it cannot be excluded that some endoparasites may also develop permanent forms or infect intermediate hosts, a part of the sample medium should also be dried and stored if possible. For rotifers from plankton or (slide growth) thus the water or the slides.
Many bdelloid rotifers live in mosses. These mosses can be dried in the air. These moss samples can be kept for years! |
| |
| Monogonont rotifers |
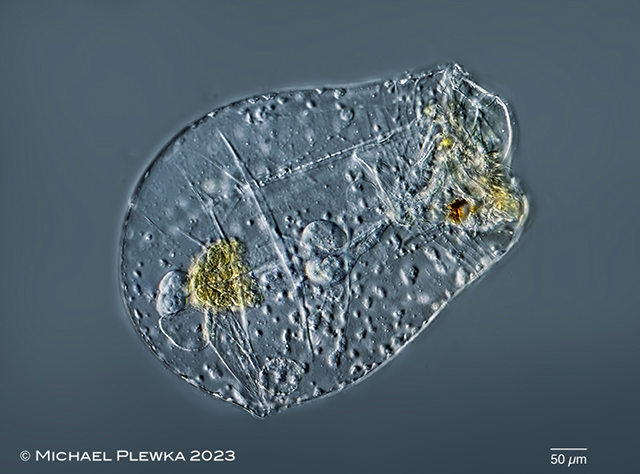 |
| Asplanchna priodonta; with unknown parasites. |
| |
 |
| Brachionus angularis; 1. infested by the fungus Zoophagus; additionally some Euglenid flagellates (>> Colacium sp.??) have colonized the integument. |
| |
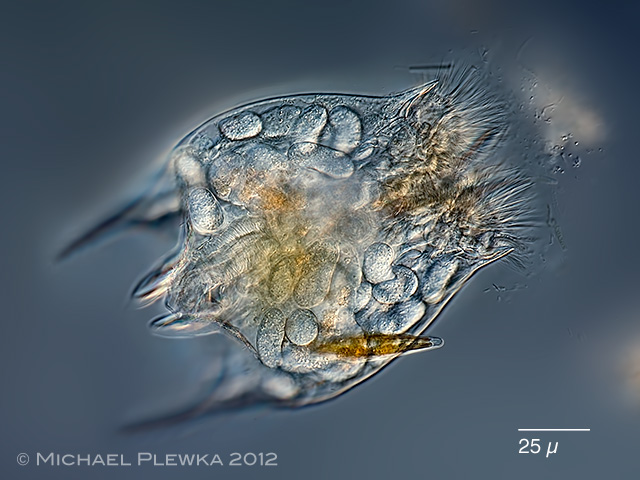 |
| Brachionus quadridentatus; infested by the Bertramia asperospora (Plistophora asperospora) |
| |
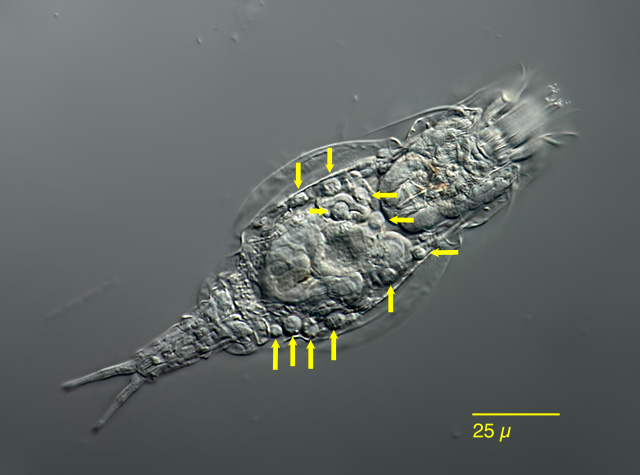 |
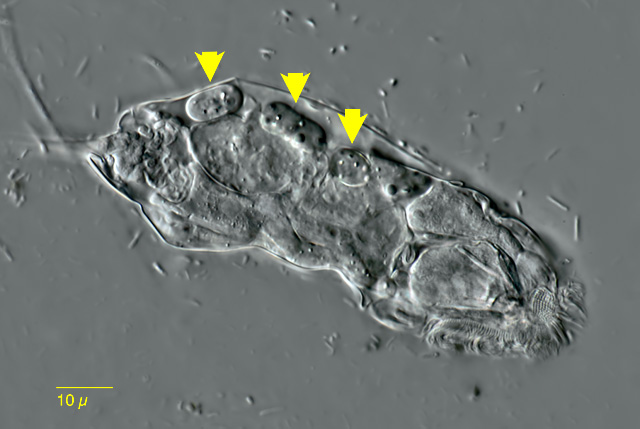
| Bryceella stylata (arrows) |
| |
 |
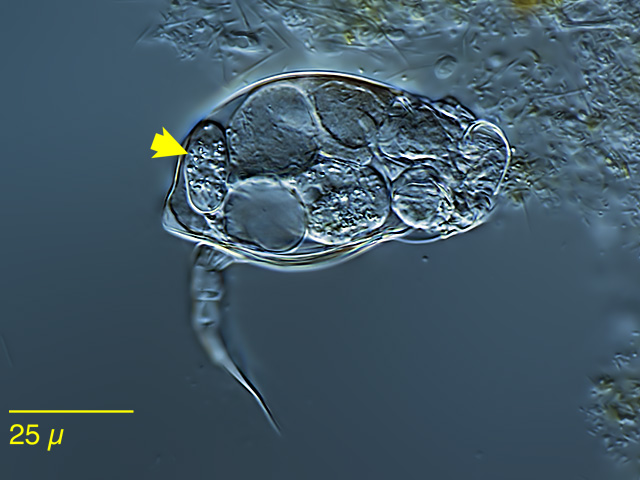
| Cephalodella bryophila(arrows) |
| |
 |
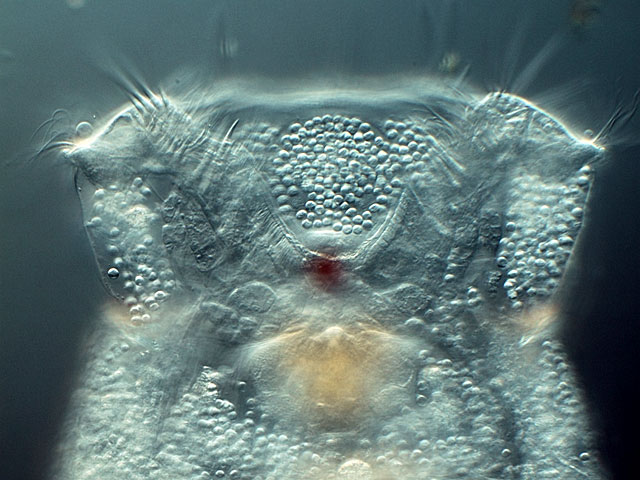
| Colurella sp. (arrow) |
| |
 |
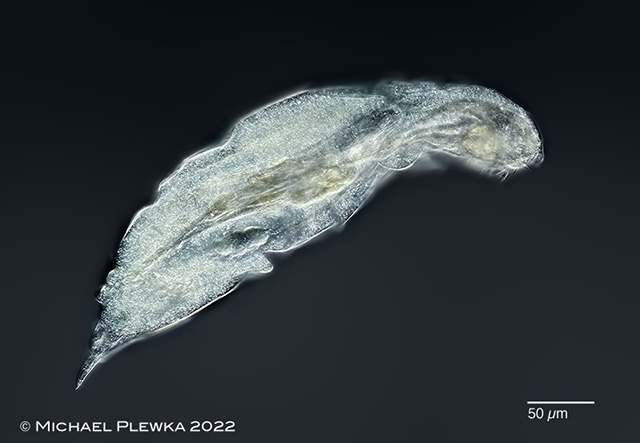
| Epiphanes brachionus |
| |
 |
| Encentrum lutra |
| |
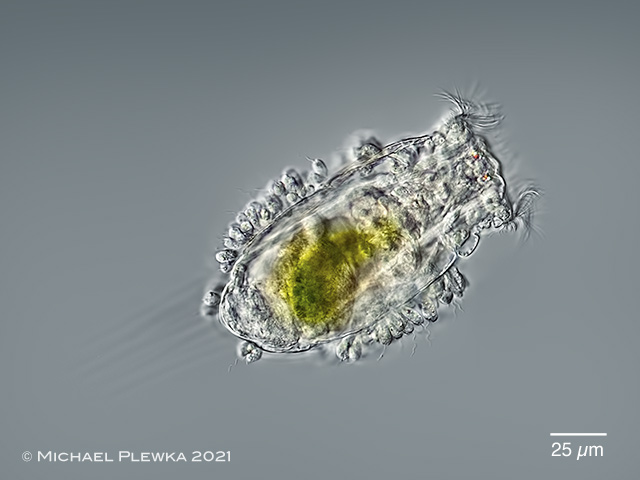 |
| Filinia longiseta |
| |
 |
| Keratella cochlearis; infested by the euglenoid flagellate Colacium vesiculosum |
| |
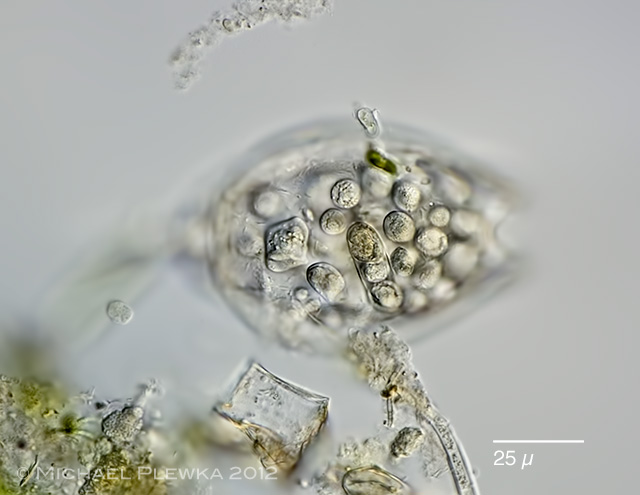 |
| Lecane sp. 02.08.2012 Sima Moor |
| |
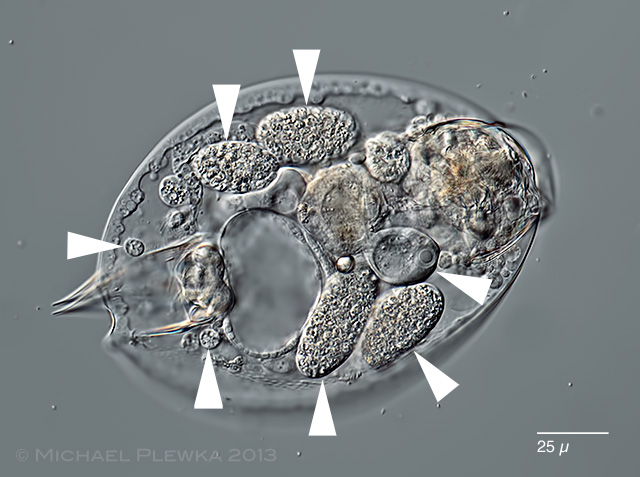 |
| Lepadella ovalis with Bertramia asperospora |
| |
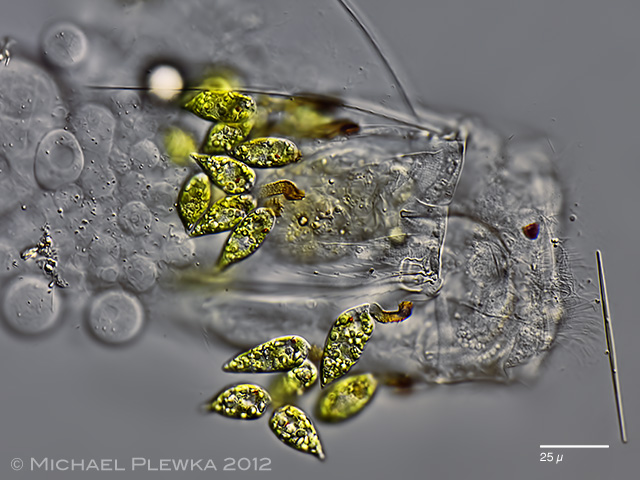 |
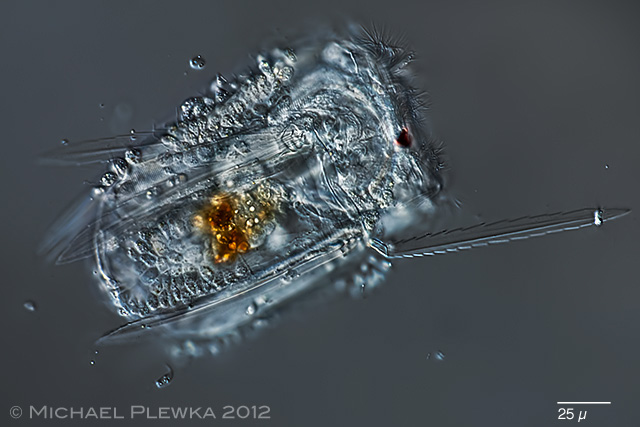
| Polyarthra vulgaris covered with euglenid flagellates Colacium sp., which may be considered as ectoparasites. |
| |
 |
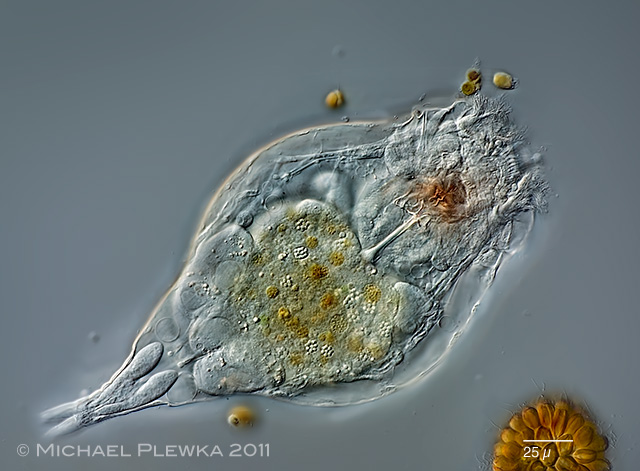
| Polyarthra vulgaris covered with zooflagellates, which may be considered as ectoparasites. |
| |
 |
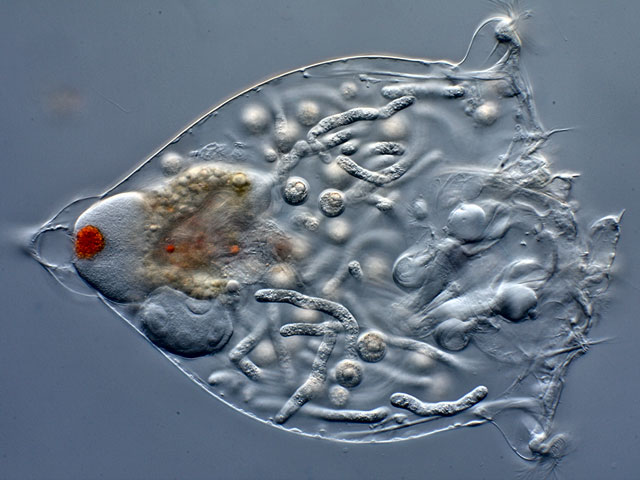
| Resticula gelida, specimen infested by parasites. (1) |
| |
 |
| Synchaeta pectinata with parasite infestation by Plistophora asperospora |
| |
| |
| |
| Bdelloid (digonont) rotifers |
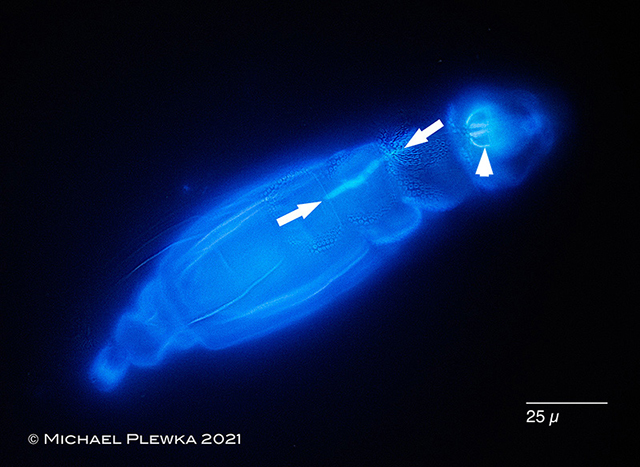 |
| Adineta tuberculosa infested by fungal parasites (marked by arrows). Specimen stained with Calcofluor (Epifluorescence). |
| |
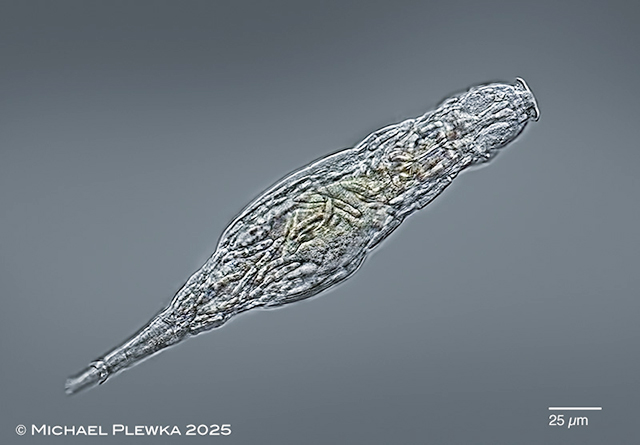 |
| Adineta rhomboidea infested by fungal parasites (Triacutus sp.). |
| |
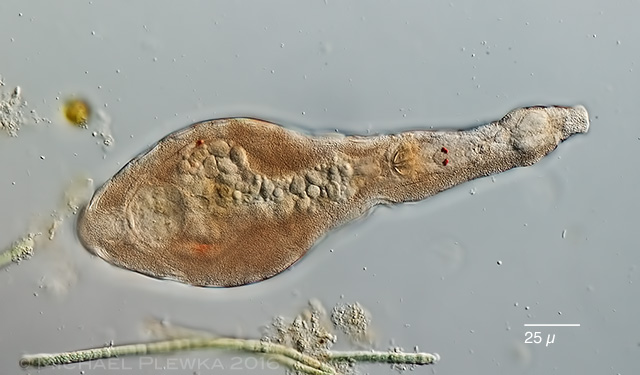 |
| Habrotrocha collaris infested by bacteria |
| |
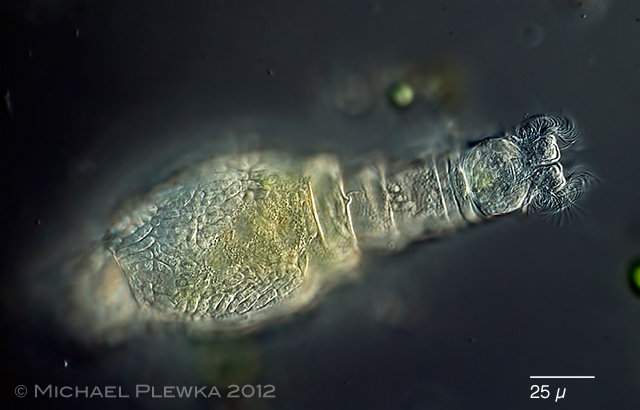 |
| Habrotrocha constricta infested by Triacutus sp. 23.12.2012 |
| |
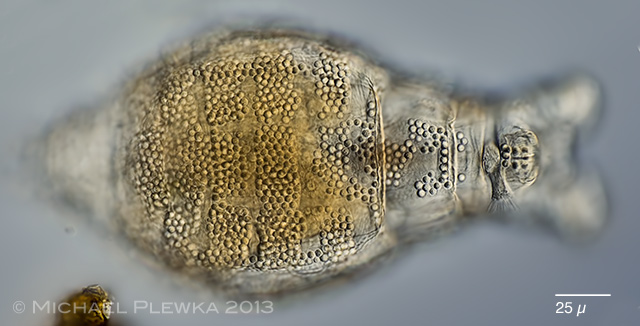 |
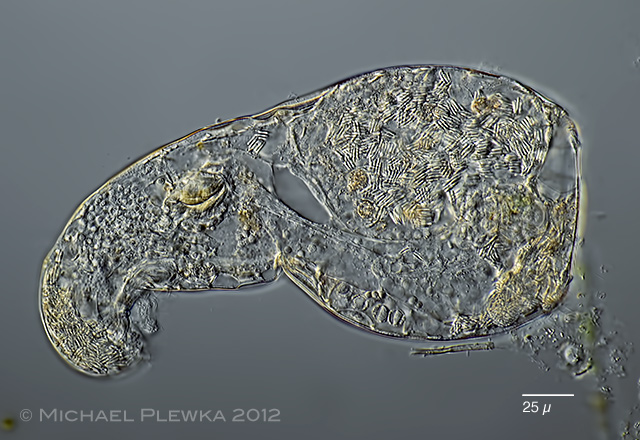
| Macrotrachela quadricornifera 29.06.2013 |
| |
 |
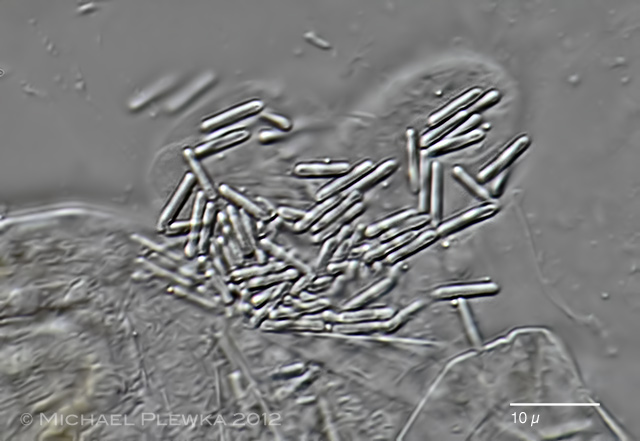 |
| Macrotrachela quadricornifera with unknown parasite 29.06.2013 |
| |
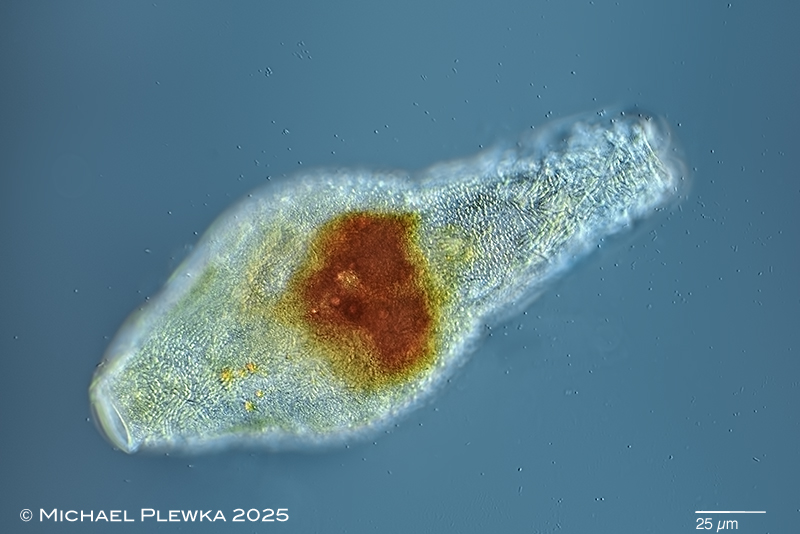 |
| Mniobia scarlatina with unknown fungal parasites (?? Triacutus sp. ??). |
| |
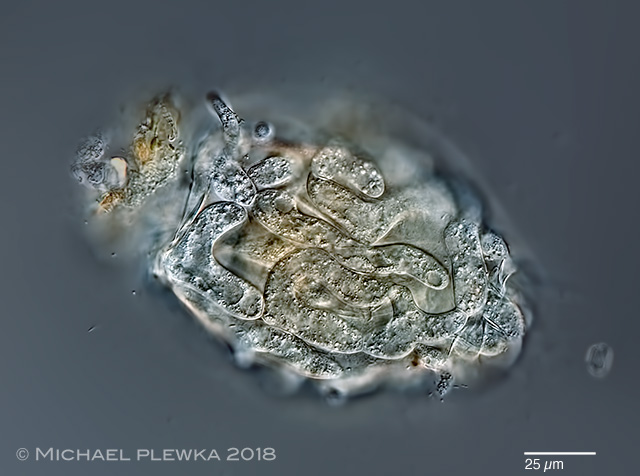 |
| Philodina cf flaviceps with unknown parasite 29.06.2013 |
| |
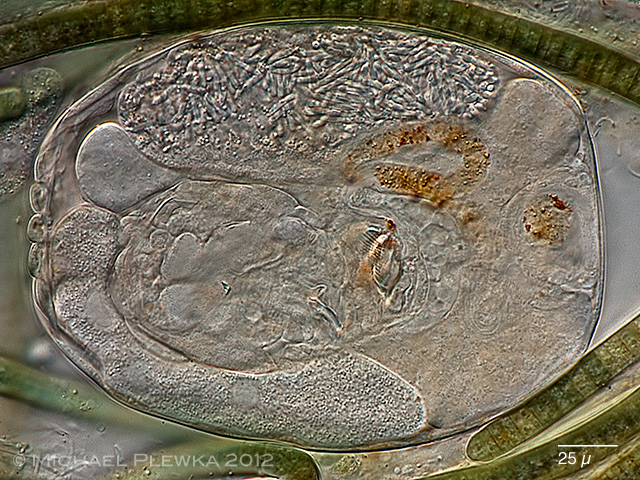 |
| Philodina tranquilla with unknown parasite 29.06.2013 |
| |
 |
| Rotaria macrura with Leptoclava parasita |
| |
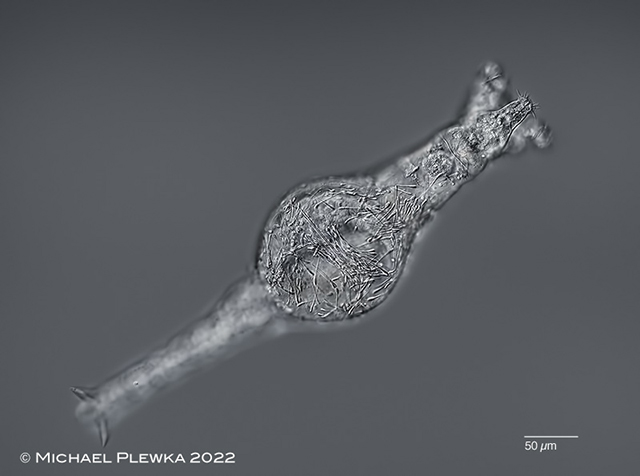 |
| Rotaria magnacalcarata with Leptoclava parasita |
| |
| |
| |
|
|
| |
| |
| |